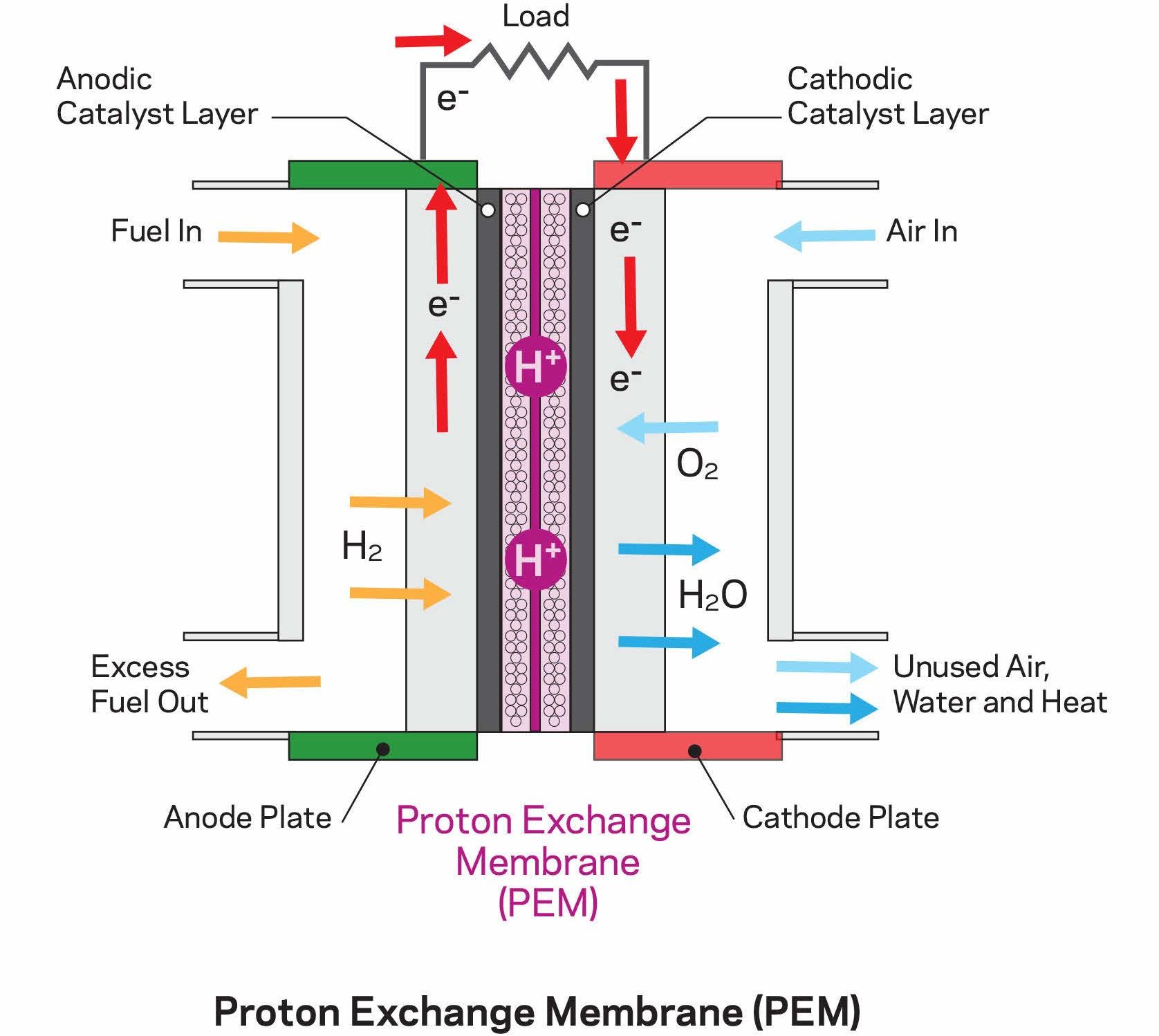
What Is The Purpose Of Creating A Reaction In A Fuel Cell . When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. In a polymer electrolyte membrane fuel cell, a catalyst. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. here's what happens in the fuel cell: a type of galvanic cell which promises to become increasingly important in the future is the fuel cell.
from www.nafion.com
When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. In a polymer electrolyte membrane fuel cell, a catalyst. a type of galvanic cell which promises to become increasingly important in the future is the fuel cell. here's what happens in the fuel cell: a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity.
Innovative Fuel Cells Enabled by Ion Exchange Membranes
What Is The Purpose Of Creating A Reaction In A Fuel Cell here's what happens in the fuel cell: in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. a type of galvanic cell which promises to become increasingly important in the future is the fuel cell. the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a polymer electrolyte membrane fuel cell, a catalyst. a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. here's what happens in the fuel cell: When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas.
From www.nafion.com
Innovative Fuel Cells Enabled by Ion Exchange Membranes What Is The Purpose Of Creating A Reaction In A Fuel Cell a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. a type of galvanic cell which. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working What Is The Purpose Of Creating A Reaction In A Fuel Cell here's what happens in the fuel cell: a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. a. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.researchgate.net
Electrochemical reactions in a fuel cell with (A) H 2 O 2 and (B) CH 4 What Is The Purpose Of Creating A Reaction In A Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. outline of the relations between a fuel cell’s principals of operation, advantages and features, and. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint What Is The Purpose Of Creating A Reaction In A Fuel Cell a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. In a polymer electrolyte membrane fuel cell, a catalyst. here's what happens in the fuel cell: outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. a fuel, such as hydrogen,. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink What Is The Purpose Of Creating A Reaction In A Fuel Cell When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. in an exothermic chemical reaction the electrons or the electrical. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.vedantu.com
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell. What Is The Purpose Of Creating A Reaction In A Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst. a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy What Is The Purpose Of Creating A Reaction In A Fuel Cell in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. here's what happens in the fuel cell: In a polymer electrolyte membrane fuel cell, a catalyst. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. a type of galvanic cell which promises to. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.researchgate.net
1 Fuel Cell Reaction through and external circuit, thus creating What Is The Purpose Of Creating A Reaction In A Fuel Cell outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. here's what happens in the fuel cell: the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From dxohghvna.blob.core.windows.net
Fuel Cell Catalyst Technology at Sarah Ables blog What Is The Purpose Of Creating A Reaction In A Fuel Cell a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity. here's what happens. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.chfca.ca
About Fuel Cells CHFCA What Is The Purpose Of Creating A Reaction In A Fuel Cell a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. When hydrogen gas pumped from the fuel tanks arrives. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources What Is The Purpose Of Creating A Reaction In A Fuel Cell a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. here's what happens in the fuel cell: in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. outline of the relations between. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica What Is The Purpose Of Creating A Reaction In A Fuel Cell a type of galvanic cell which promises to become increasingly important in the future is the fuel cell. When hydrogen gas pumped from the fuel tanks arrives at the anode, which is. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. outline of the relations between a fuel cell’s principals of. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From semiengineering.com
Fuel Cells And The IoE What Is The Purpose Of Creating A Reaction In A Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst. the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity. here's what happens in the fuel cell: a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. a type of. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From courses.lumenlearning.com
Phases and Classification of Matter Chemistry Atoms First What Is The Purpose Of Creating A Reaction In A Fuel Cell here's what happens in the fuel cell: the basic working principle of a fuel cell involves the reaction of hydrogen and oxygen to produce water and electricity. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. in an exothermic chemical reaction the electrons or the electrical charges are. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.science-revision.co.uk
Fuel Cells What Is The Purpose Of Creating A Reaction In A Fuel Cell outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. In a polymer electrolyte membrane fuel cell, a catalyst. a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. a fuel cell is a device that generates electricity through an electrochemical reaction, not. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From large.stanford.edu
Hydrogen Fuel Cells What Is The Purpose Of Creating A Reaction In A Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst. outline of the relations between a fuel cell’s principals of operation, advantages and features, and main areas. a fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. here's what happens in the fuel cell: the basic working principle of a. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From china.denora.com
Anodes and Cathodes for Fuel Cells De Nora What Is The Purpose Of Creating A Reaction In A Fuel Cell a type of galvanic cell which promises to become increasingly important in the future is the fuel cell. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. a fuel cell reaction as an electrochemical reaction involves the transfer of electrons (charges) that occurs between an. a fuel, such as hydrogen,. What Is The Purpose Of Creating A Reaction In A Fuel Cell.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel What Is The Purpose Of Creating A Reaction In A Fuel Cell a type of galvanic cell which promises to become increasingly important in the future is the fuel cell. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. in an exothermic chemical reaction the electrons or the electrical charges are directly exchanged between. here's what happens in the fuel cell: . What Is The Purpose Of Creating A Reaction In A Fuel Cell.